Abstract
BACKGROUND: The discovery of adenosine triphosphate (ATP)-competitive TKIs has extended life expectancy in CML; despite these advances, 28% to 40% of newly diagnosed pts with CML-CP discontinue treatment by 5 years, prompting a switch to second-line (2L) therapy. Discontinuation rates increase in 2L; up to 86% of pts discontinue therapy, with 20% to 30% discontinuing 2L therapy due to disease progression. Dose-escalation studies show that some pts experiencing resistance to therapy can achieve responses with higher doses of their TKI, while use of another second-generation (2G) TKI has shown limited clinical benefit in pts experiencing resistance to a 2G TKI. Asciminib is the 1st BCR::ABL1 inhibitor that Specifically Targets the ABL Myristoyl Pocket (STAMP), allowing asciminib to have activity against most BCR::ABL1 mutations that impart resistance to ATP-competitive TKIs. Asciminib was 1st approved in the United States for pts with CML-CP after ≥2 TKIs at 80 mg once daily (QD) and 40 mg twice daily (BID) and CML-CP with the T315I mutation at 200 mg BID.
In the phase 3 ASCEMBL study, asciminib 40 mg BID showed superior efficacy vs bosutinib 500 mg QD in pts with CML-CP after ≥2 prior TKIs at weeks 24 (major molecular response [MMR], 25.5% vs 13.2%) and 96 (MMR, 37.6% vs 15.8%) and favorable tolerability with >2 years’ follow-up. In a large phase 1 dose-finding trial, asciminib demonstrated safety across doses of 80 to 200 mg QD and 10 to 200 mg BID in pts with CML-CP/accelerated phase without and with the T315I mutation previously treated with ≥2 and ≥1 TKIs, respectively (maximum tolerated dose was not reached in this study). Here, we describe the phase 2 ASC2ESCALATE trial investigating the efficacy and safety of 2L asciminib with dose escalation in pts with CML-CP (NCT05384587).
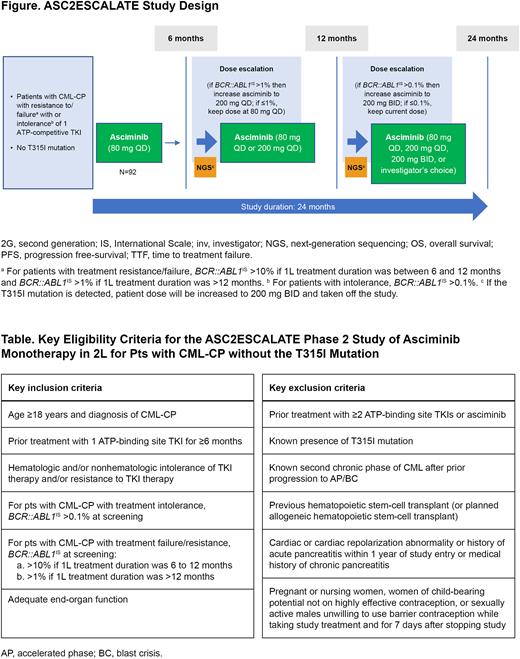
STUDY DESIGN AND METHODS: This is a phase 2, open-label, multicenter, single-arm, dose-escalation study in the United States (Figure). Adult pts (aged ≥18 years) with CML-CP without the T315I mutation who experienced resistance (BCR::ABL1IS >1% with 6-12 months of first-line [1L] treatment or >10% with >12 months of 1L treatment) or intolerance (BCR::ABL1IS >0.1%) with ≥6 months of treatment with 1 prior ATP-competitive TKI are eligible (Table). All pts will initiate treatment with asciminib 80 mg QD. For pts not achieving BCR::ABL1IS <1% at 6 months, dose will be escalated to 200 mg QD if pts do not have grade ≥3 toxicity or persistent grade 2 toxicity refractory to optimal management. In pts not achieving MMR at 12 months, either dose escalation from 80 to 200 mg QD or from 200 mg QD to 200 mg BID will occur or the pts will discontinue study treatment. Pts who achieve MMR at 12 months will continue asciminib at their current dose. Pts deriving clinical benefit from asciminib per investigator assessment may receive post-trial access. Pts may discontinue at any time due to unacceptable toxicity or progression or at investigator/pt discretion.
The primary endpoint is the proportion of pts achieving MMR at 12 months. Secondary endpoints include MMR rates by 3, 6, 18, and 24 months; MR4.5 (BCR::ABL1IS ≤0.0032%) at 24 months; time to and duration of MMR; time to treatment failure; and safety/tolerability. To investigate progression-free and overall survival, time from the first dose to progression or death due to any cause during the study, respectively, will be measured.
CONCLUSIONS: ASC2ESCALATE is the 1st clinical trial designed to assess asciminib in pts with CML-CP requiring 2L therapy and seeks to provide information on the efficacy and safety of dose escalation for pts not meeting certain response milestones. Treatment using a dose escalation strategy with asciminib in 2L could enable a greater proportion of pts with CML-CP not meeting milestones to achieve molecular responses and provide another option for those experiencing intolerance of or resistance to ATP-competitive TKIs. Recruitment for this study is planned to begin August 2022 with an approximate enrollment of 92 pts with CML-CP across ≈40 sites.
This study is sponsored by Novartis.
Disclosures
Sasaki:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees. Mauro:Sun Pharma/SPARC: Research Funding; AbbVie, Bristol Myers Squibb, Novartis, Pfizer, Takeda: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding. Levy:Abbvie: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Karyopharm: Honoraria, Speakers Bureau; Morphosys: Honoraria, Speakers Bureau; Seagen: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; AstraZeneca: Honoraria, Speakers Bureau; BeiGene: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Sellas: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Speakers Bureau; Dova: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau; Epizyme: Honoraria, Speakers Bureau; TG Therapeutics: Honoraria, Speakers Bureau; Baylor University Medical Center: Current Employment. Atallah:Abbvie: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Takeda: Research Funding; Novartis: Consultancy, Research Funding; Blueprint: Speakers Bureau. Koller:Treadwell Therapeutics: Other: Safety Review Committee; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau. Maegawa:Novartis: Current Employment. Damon:Novartis: Current Employment, Current equity holder in publicly-traded company. Kumar:Novartis: Current Employment. Khan:Novartis: Current Employment, Current equity holder in publicly-traded company. Cortes:Pfizer: Consultancy, Honoraria, Research Funding; Forma Therapuetic: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Sun Pharma: Consultancy, Research Funding; Gilead: Consultancy; Biopath Holdings: Consultancy, Current equity holder in private company; Novartis: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Kartos: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal